New research suggests booster dose of Novavax NVX-CoV2373 vaccine is effective against SARS-CoV-2 Omicron subvariants
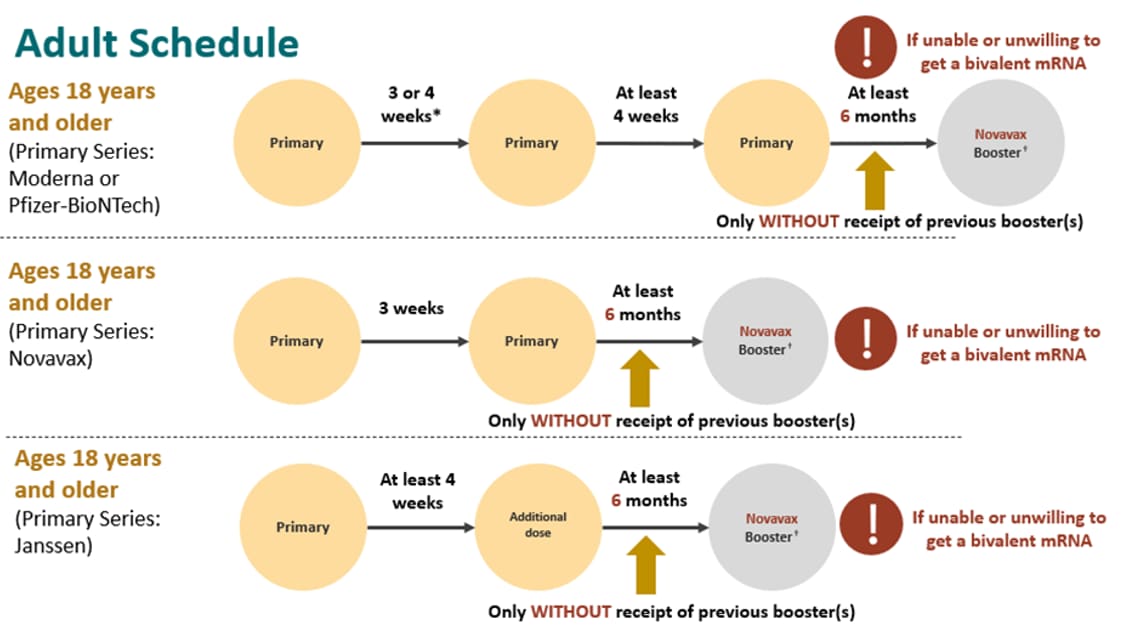
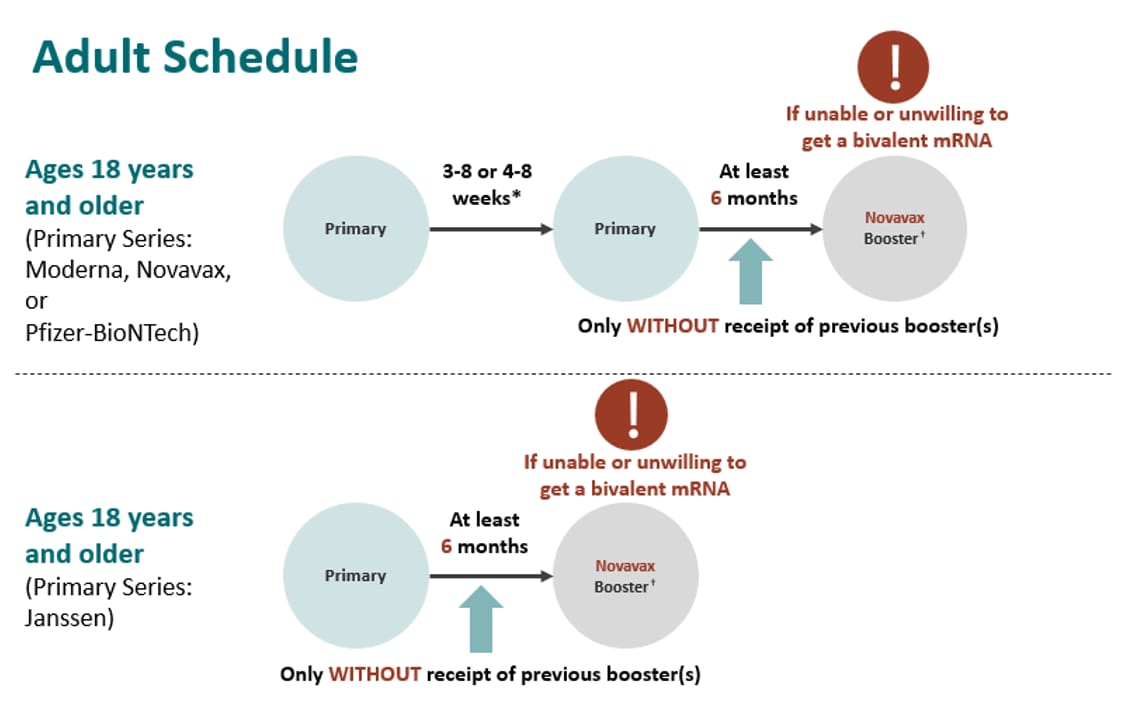
ACIP Evidence to Recommendations (EtR) for Use of Novavax COVID-19 Vaccine Booster Dose for adults ages 18 years and older under an Emergency Use Authorization | CDC
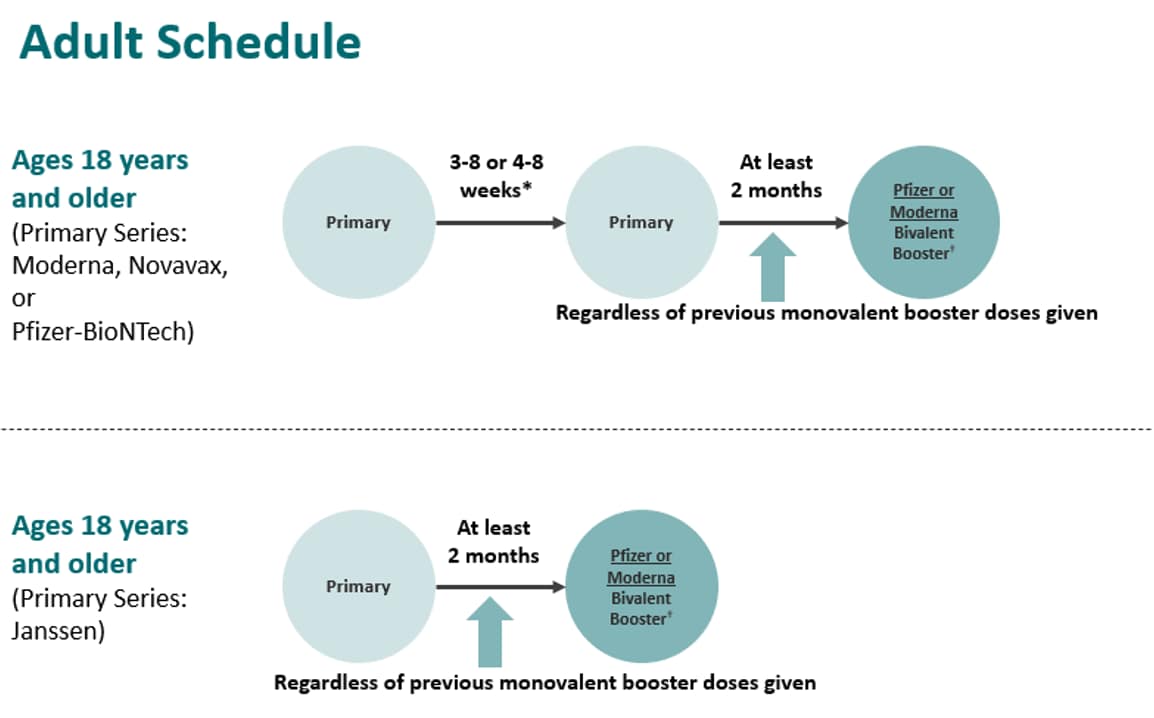
ACIP Evidence to Recommendations (EtR) for Use of Novavax COVID-19 Vaccine Booster Dose for adults ages 18 years and older under an Emergency Use Authorization | CDC

Nobody wants to speak about COVID': Less than 3% of eligible Americans got a booster shot in September — does that mean trouble for these 3 big vaccine stocks?
Booster e terza dose, che differenza c'è? Cosa cambia ed ecco perché il termine inglese è usato a sproposito

Novavax Expects Its Updated COVID-19 Vaccine Candidate to Work on Circulating Variants | Health News | U.S. News









/cloudfront-us-east-1.images.arcpublishing.com/gray/COUI7Y75YBEDLGZLB356FFMMC4.jpg)