BioNTech SE on X: "+++ Omicron BA.4/BA.5 vaccine update +++ The FDA today granted Emergency Use Authorization (EUA) of a 30-µg booster dose of our Omicron BA.4/BA.5 bivalent vaccine, as a booster

News - CHMP Recommends Additional Authorisation Modification of Comirnaty (BioNTech/Pfizer) As a Bivalent Vaccine Adapted to Omicron BA.4/BA.5 for Booster Vaccinations - Paul-Ehrlich-Institut

ATAGI recommendations on use of the Pfizer bivalent (Original/Omicron BA.4/5) COVID-19 vaccine | Australian Government Department of Health and Aged Care
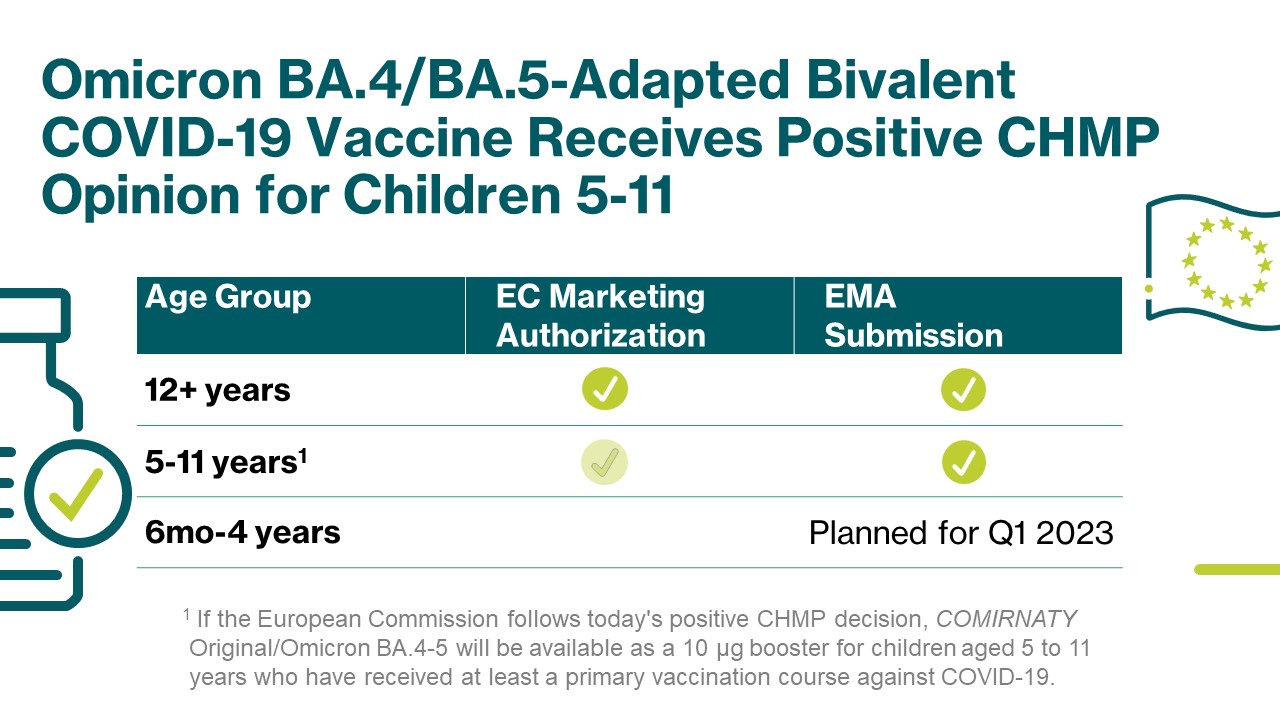
BioNTech SE on X: "A booster dose of our Omicron BA.4/BA.5-adapted bivalent #COVID19 vaccine developed with @Pfizer has been recommended for marketing authorization by the @EMA_News' #CHMP for children 5-11. https://t.co/COyxj9fKS4 https://t.co ...

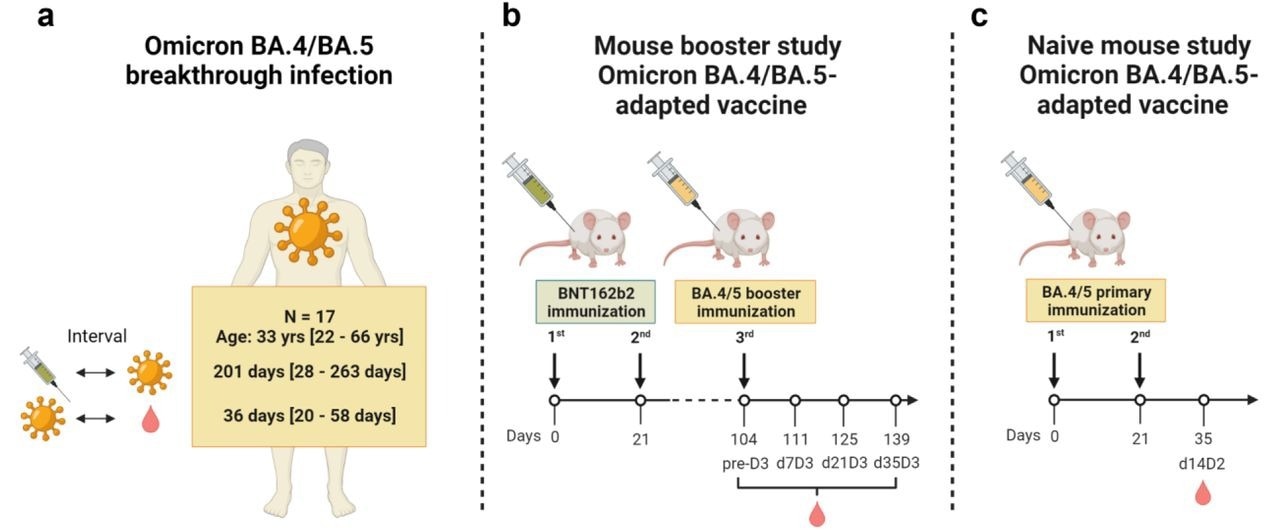
BioNTech SE on X: "We announced today with @Pfizer updated 30-day data for the 30-µg booster dose of our Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine. https://t.co/h2ZEfBpuNb https://t.co/oaOxsYgPSk" / X
FDA vaccine advisers 'disappointed' and 'angry' that early data about new Covid-19 booster shot wasn't presented for review last year | CNN

BioNTech SE on X: "We completed, together with @Pfizer, the submission to the @US_FDA requesting Emergency Use Authorization (EUA) of a 10-µg booster dose of the Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine for
CDC recommends new COVID booster for all Americans over 6 months amid rising cases, hospitalizations - ABC News