Chemical structure, dipole moment δ, in D, and Gutmann's donor number... | Download Scientific Diagram
Alcohols Alcohol – any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosm

Influence of dipole moments on the medicinal activities of diverse organic compounds - ScienceDirect
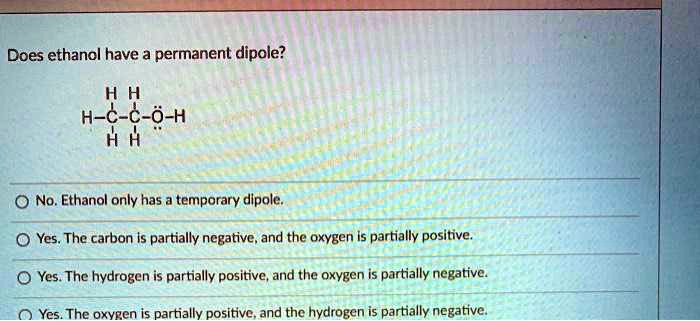
SOLVED: Does ethanol have a permanent dipole? H-C-C-O-H H H No. Ethanol only has a temporary dipole. Yes. The carbon is partially negative, and the oxygen is partially positive. Yes. The hydrogen

Provide the following information for CH3CH2OH. a. Lewis dot structure b. bond polarity (show dipole vectors) c. molecular polarity d. identify all intermolecular forces present | Homework.Study.com

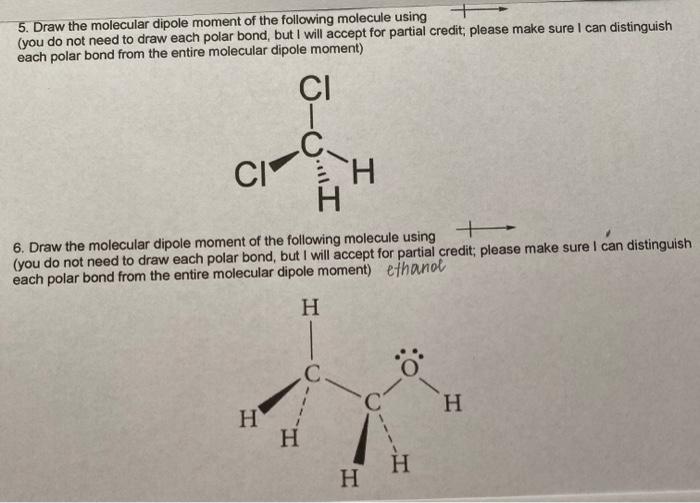
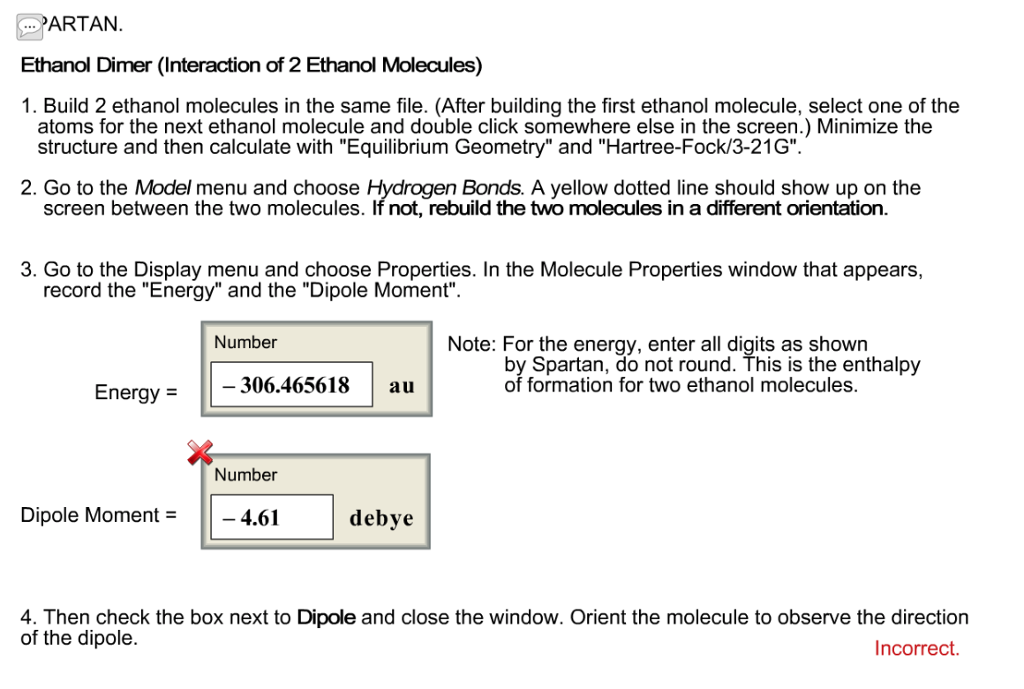
Predict the direction of the net molecular dipole for ethanol, as well as predict the shape around each carbon and nitrogen atom in dimethylformamide, and predict whether the molecule is polar or