
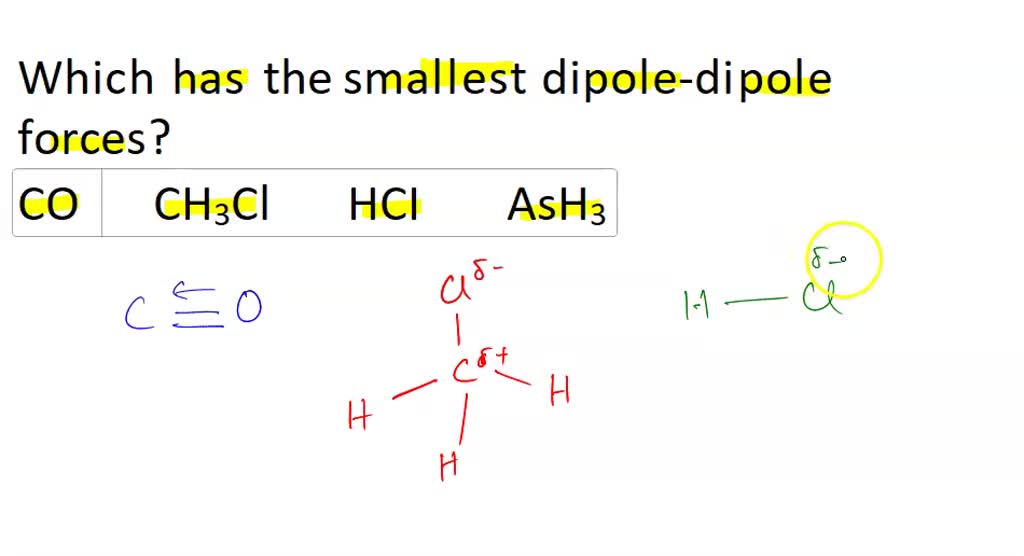
Which of the following orders is correct?(1) SbH_{3}> NH_{3}> AsH_{3}> PH_{3} - Boilling point(2) NH_{3}> PH_{3}> AsH_{3}> SbH_{3} - Thermal stability(3) NH_{3}> PH_{3}> AsH_{3}> SbH_{3} - Basic character(4) NH_{3}> PH_{3}> AsH_{3}> SbH_{3} - Bond angle

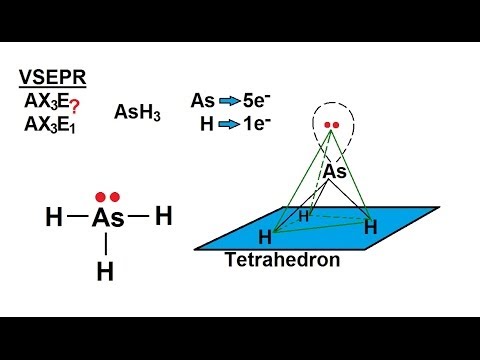
SOLVED: AsH3 vs ClF3. what are the Lewis structure and resonance? Why? What are the electronic geometry? Shape? Bond angles? Polar or nonpolar? why? Hybridization?

Simulating Vapor–Liquid Equilibria of PH3, AsH3, and SbH3 from First Principles | The Journal of Physical Chemistry C

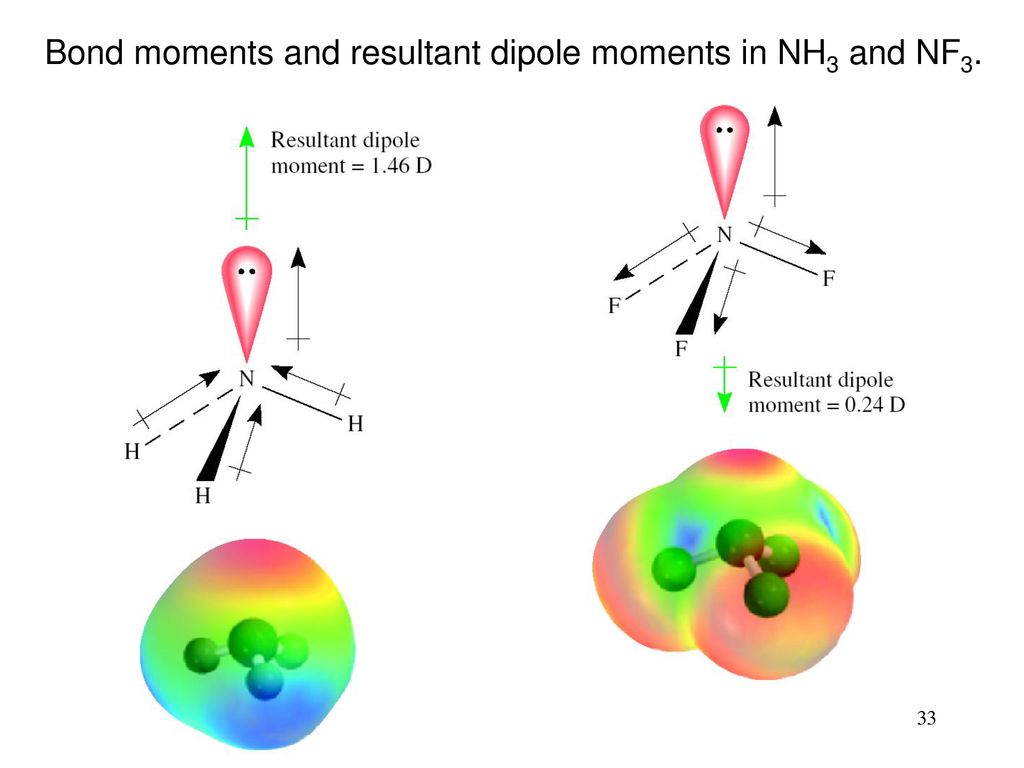
Which of the following substances have permanent dipole-dipole forces? GeH4; molecular MgCl2; PI3; F2O | Homework.Study.com
✓ Solved: Which of the following molecules or ions are trigonal planar? a. AsH3 b. TeF5^- c. BBr3 d....

Of the molecules listed, which does not have a dipole moment? a. HCl b. NCl3 c. CO d. BF3 e. All molecules have a dipole moment. | Homework.Study.com

u ule mass of one mole of electrons. JH 40 respectively. Arrange the following is increasing order of property given () O,F,S, CI, N strength of H-bonding (X-H-X). Secil (ii) N2, 02,


![PDF] The dipole moment of the C—H bond by C. A. Coulson · 10.1039/tf9423800433 · OA.mg PDF] The dipole moment of the C—H bond by C. A. Coulson · 10.1039/tf9423800433 · OA.mg](https://og.oa.mg/The%20dipole%20moment%20of%20the%20C%E2%80%94H%20bond.png?author=%20C.%20A.%20Coulson)







![PDF] Absolute local mode vibrational band intensities of AsH3 | Semantic Scholar PDF] Absolute local mode vibrational band intensities of AsH3 | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5609af77a8c2ebdb97a2c432d6643b66067182f6/5-Table3-1.png)




![PDF] Absolute local mode vibrational band intensities of AsH3 | Semantic Scholar PDF] Absolute local mode vibrational band intensities of AsH3 | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5609af77a8c2ebdb97a2c432d6643b66067182f6/3-Figure1-1.png)